Transcriptome Analysis of Drought-Tolerant Mechanisms in Mutant Chili
DOI:
https://doi.org/10.48048/tis.2024.7934Keywords:
CaWDT-2, Proline, Drought-tolerant chili, Gene expressionAbstract
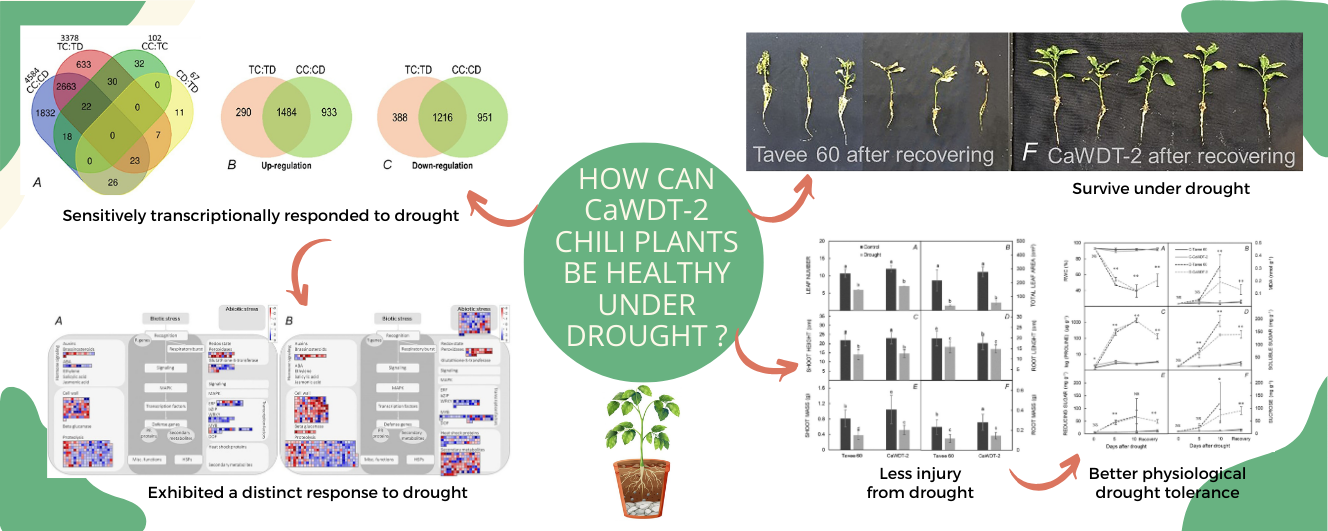
Drought is a natural disaster that negatively impacts agricultural crops. Understanding the mechanisms of water deficit response is key to improving drought-tolerant crops. This study focused on comprehending the drought-tolerant mechanisms of a mutant chili, CaWDT-2, by investigating its physiological phenotypes and gene expression under PEG-induced drought condition compared to Tavee 60 as the original cultivar. Chili physiological status was monitored by shoot height, root length, leaf number, leaf area, shoot mass and root mass at 10 d after drought initiation. Relative water content (RWC), malondialdehyde (MDA), proline, soluble sugar and sucrose were measured during drought and after recovery. Transcriptome analysis using sequencing of RNA (RNA-seq) was employed to examine gene expression profiling under drought for 24 h. Results revealed diverse changes in physiological mechanism in response to drought between CaWDT-2 and Tavee 60 cultivars. Comparative transcriptomic analysis is helpful in understanding the differences in drought tolerance mechanisms between 2 crop varieties. Major differences that emerged from the physiological and transcriptomic analyses included drought-stressed injury level, proline accumulation, root cell wall adaptation, ABA biosynthesis, stress detoxification, heat shock proteins and transcription factors. Our data will contribute to further research in developing drought-tolerant crops. An improved understanding of drought tolerance mechanisms will enhance agricultural resilience and mitigate the impact of drought on crop yield.
HIGHLIGHTS
- CaWDT-2, a drought-tolerant chili, could recover after exposure to a 10-d, 15 % PEG-induced drought, while Tavee 60, a drought-sensitive chili, could not
- Physiological studies indicate that CaWDT-2 displayed higher tolerance to drought than Tavee 60
- The roots of CaWDT-2 exhibited distinct drought-tolerant mechanisms at the gene expression level compared to Tavee 60 roots 24-h after water deficit
- Comparative transcriptomic analysis is helpful in understanding the differences in drought tolerance mechanisms between CaWDT-2 and Tavee 60
GRAPHICAL ABSTRACT
Downloads
References
G Matmarurat. 2022, The physiological characteristics and gene expression of drought-tolerant chili from the mutant induction by gamma radiation. Ph. D. Dissertation. Kasetsart University, Bangkok, Thailand.
A Dudhate, H Shinde, D Tsugama, S Liu and T Takano. Transcriptomic analysis reveals the differentially expressed genes and pathways involved in drought tolerance in pearl millet [Pennisetum glaucum (L.) R. Br]. PloS One 2018; 13, e0195908.
A Fracasso, LM Trindade and S Amaducci. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 2016; 16, 115.
G Villanueva, S Vilanova, M Plazas, J Prohens and P Gramazio. Transcriptome profiles of eggplant (Solanum melongena) and its wild relative S. dasyphyllum under different levels of osmotic stress provide insights into response mechanisms to drought. Curr. Plant Biol. 2023; 33, 100276.
L González and M González-Vilar. Determination of relative water content. In: MJR Roger (Ed.). Handbook of plant ecophysiology techniques. Kluwer Academic Publishers, Dordrecht, Netherlands, 2001, p. 207-12.
J Zhang and MB Kirkham. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist 1996; 132, 361-73.
H Koca, M Bor, F Özdemir and İ Türkan. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007; 60, 344-51.
EW Yemm and AJ Willis. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954; 57, 508-14.
GL Miller. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959; 31, 428-8.
CA Schneider, WS Rasband and KW Eliceiri. NIH image to ImageJ: 25 years of image analysis. Nat. Meth. 2012; 9, 671-5.
M Lohse, A Nagel, T Herter, P May, M Schroda, R Zrenner, T Tohge, AR Fernie, M Stitt and B Usadel. Mercator: A fast and simple web server for genome scale functional annotation of plant sequence data. Plant Cell Environ. 2014; 37, 1250-8.
T Zaher-Ara, N Boroomand and M Sadat-Hosseini. Physiological and morphological response to drought stress in seedlings of ten citrus. Trees 2016; 30, 985-93.
Y Du, Q Zhao, L Chen, X Yao, W Zhang, B Zhang and F Xie. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020; 146, 1-12.
MJ O’Brien, A Valtat, S Abiven, MS Studer, R Ong and B Schmid. The role of soluble sugars during drought in tropical tree seedlings with contrasting tolerances. J. Plant Ecol. 2020; 13, 389-97.
DC Dien, T Mochizuki and T Yamakawa. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019; 22, 530-45.
G Matmarurat, K Chutinanthakun, P Juntawong and O Khamsuk. Two distinct mechanisms of water and energy conservation confer drought tolerance in chili mutants. Acta Physiologiae Plantarum 2022; 44, 7.
A Soares, S Niedermaier, R Faro, A Loos, B Manadas, C Faro, PF Huesgen, AY Cheung and I Simões. An atypical aspartic protease modulates lateral root development in Arabidopsis thaliana. J. Exp. Bot. 2019; 70, 2157-71.
A Kohli, JO Narciso, B Miro and M Raorane. Root proteases: Reinforced links between nitrogen uptake and mobilization and drought tolerance. Physiol. Plant. 2012; 145, 165-79.
TM Nolan, N Vukasinovic, D Liu, E Russinova and Y Yin. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020; 32, 295-318.
GJ Ahammed, X Li, A Liu and S Chen. Brassinosteroids in plant tolerance to abiotic stress. J. Plant Growth Regul. 2020; 39, 1451-64.
K Shinozaki and K Yamaguchi-Shinozaki. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007; 58, 221-7.
UJ Phukan, GS Jeena, V Tripathi and RK Shukla. Regulation of Apetala2/ethylene response factors in plants. Front. Plant Sci. 2017; 8, 150.
C Gu, ZH Guo, PP Hao, GM Wang, ZM Jin and SL Zhang. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot Stud. 2017; 58, 6.
MY Shi, YT Du, J Ma, DH Min, LG Jin, J Chen, M Chen, YB Zhou, YZ Ma, ZS Xu and XH Zhang. The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int. J. Mol. Sci. 2018; 19, 4087.
M Hrmova and SS Hussain. Plant transcription factors involved in drought and associated stresses. Int. J. Mol. Sci. 2021; 22, 5662.
M Wu, K Zhang, Y Xu, L Wang, H Liu, Z Qin and Y Xiang. The moso bamboo WRKY transcription factor, PheWRKY86, regulates drought tolerance in transgenic plants. Plant Physiol. Biochem. 2022; 170, 180-91.
X Wang, Y Niu and Y Zheng. Multiple functions of MYB transcription factors in abiotic stress responses. Int. J. Mol. Sci. 2021; 22, 6125.
W Nawae, JR Shearman, S Tangphatsornruang, P Punpee, T Yoocha, D Sangsrakru, C Naktang, C Sonthirod, W Wirojsirasak, K Ukoskit, K Sriroth, P Klomsa-ard and W Pootakham. Differential expression between drought-tolerant and drought-sensitive sugarcane under mild and moderate water stress as revealed by a comparative analysis of leaf transcriptome. PeerJ 2020; 8, e9608.
X Liu, H Cui, B Zhang, M Song, S Chen, C Xiao, Y Tang and J Liesche. Reduced pectin content of cell walls prevents stress-induced root cell elongation in Arabidopsis. J. Exp. Bot. 2021; 72, 1073-84.
PK Das, R Biswas, N Anjum, AK Das and MK Maiti. Rice matrix metalloproteinase OsMMP1 plays pleiotropic roles in plant development and symplastic-apoplastic transport by modulating cellulose and callose depositions. Sci. Rep. 2018; 8, 2783.
W Gou, X Li, S Guo, Y Liu, F Li and Q Xie. Autophagy in plant: A new orchestrator in the regulation of the phytohormones homeostasis. Int. J. Mol. Sci. 2019; 20, 2900.
HCJ van Rensburg, WV den Ende and S Signorelli. Autophagy in plants: Both a puppet and a puppet master of sugars. Front. Plant Sci. 2019; 10, 14.
G Reyt, P Ramakrishna, I Salas-Gonzalez, S Fujita, A Love, D Tiemessen, C Lapierre, K Morreel, M Calvo-Polanco, P Flis, N Geldner, Y Boursiac, W Boerjan, MW George, G Castrillo and DE Salt. Two chemically distinct root lignin barriers control solute and water balance. Nat. Comm. 2021; 12, 2320.
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2024 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.






