The Comparison between Energy Density of Blue and Red Light which Activation Silver Nanoparticles to Inhibition Candida albicans Biofilms
DOI:
https://doi.org/10.48048/tis.2024.7702Keywords:
Silver nanoparticles, Red-blue LEDs, Photoinactivation, Candida albicans biofilms, XTT assay, Malondialdehyde, Lipid lysisAbstract
Photodynamic inactivation (PDI) is a technique to inhibit microbial biofilm growth through the toxicity of Reactive Oxygen Species (ROS) compounds. ROS can be attack membrane, lipids, DNA and nucleic acid then initiate cell necrosis. This study aims to analyze the potential of red and blue LEDs to activating silver nanoparticles (AgNPs) to produce significant amounts of ROS that are believed to be toxic and lethal to Candida albicans biofilm cells. The effectiveness of the treatment in this study was evaluated through cell viability represented by Optical Density values and malondialdehyde levels. There were 4 treatment groups used as samples, namely the control group, the photosensitizer group, the light group, and the combination group of light with photosensitizer. The duration of light exposure ranged from 2 to 10 min with a power of 100 MW. The biofilm staining done to detection some indicator as an impact of photodynamic against mortality and survive cell with 2 dyes are XTT assay as cell viability values and the Thiobarbituric Acid Reactive Substances assay for malondialdehyde levels. The results showed that photoinactivation of Candida albicans biofilm with the lowest viability occurred in the treatment group of the combination of blue light with AgNPs with an irradiation duration of 10 min, namely 0.076 ± 0.005 and the treatment group of the combination of red light with AgNPs with an irradiation duration of 10 min, namely 0.131 ± 0.021. The data resulted in an inactivation rate of 94.68 ± 0.55 % for blue light and 90.98 ± 0.02 % for red light. The malondialdehyde levels were 1.563 nmol/mL for blue light and 1.514 nmol/mL for red light. The comparison of blue light treatment with red light is based on penetration in the cell, where blue light has low penetration but high energy which gives more opportunities to produce ROS at the triplet level. The combination of blue LED spectrum with AgNPs is highly effective in inactivating the metabolic activity of pathogenic microbial cells.
HIGHLIGHTS
Candida albicans biofilm is very rigid and has strong potential as a chronic infection. The research focuses on the application of photodynamic inactivation with LED light and antimicrobial AgNPs. Identification of the results with XTT assay 94.65 % inhibition and TBARS assay at MDA level of 1,864 nmol/mL.
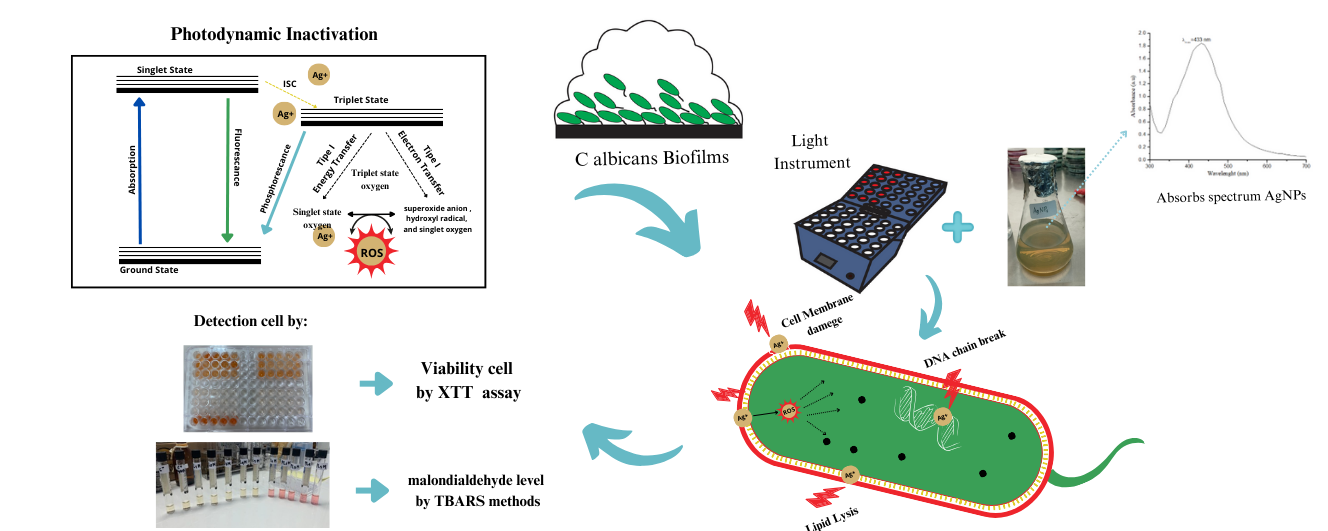
GRAPHICAL ABSTRACT

Downloads
References
J Talapko, M Juzbašić, T Matijević, E Pustijanac, S Bekić, I Kotris and I Škrlec. Candida albicans-the virulence factors and clinical manifestations of infection. J. Fungi 2021; 7, 79.
HOJ Morad, AM Wild, S Wiehr, G Davies, A Maurer, BJ Pichler and CR Thornton. Pre-clinical imaging of invasive candidiasis using ImmunoPET/MR. Front. Microbiol. 2018; 9, 1996.
T Atriwal, K Azeem, FM Husain, A Hussain, MN Khan, MF Alajmi and M Abid. Mechanistic understanding of candida albicans biofilm formation and approaches for its inhibition. Front. Microbiol. 2021; 12, 638609.
MB Lohse, M Gulati, AD Johnson and CJ Nobile. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018; 16, 19-31.
EWL Chow, LM Pang and Y Wang. From Jekyll to Hyde: The yeast-hyphal transition of Candida albican. Pathogens 2021; 10, 859.
PPD Barros, RD Rossoni, CMD Souza, L Scorzoni, JDC Fenley and JC Junqueira. Candida biofilms: An update on developmental mechanisms and therapeutic challenges. Mycopathologia 2020; 185, 415-24.
S Sharma, J Mohler, SD Mahajan, SA Schwartz, L Bruggemann and R Aalinkeel. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023; 11, 1614.
M Gulati and CJ Nobile. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microb. Infect. 2016; 18, 310-21.
R Pereira, RODS Fontenelle, EHSD Brito and SMD Morais. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2021; 131, 11-22.
T Maisch. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochemical Photobiological Sci. 2015; 14, 1518-26.
B Pucelik and JM Dąbrowski. Photodynamic inactivation (PDI) as a promising alternative to current pharmaceuticals for the treatment of resistant microorganisms. Adv. Inorg. Chem. 2022; 79, 65-103.
JM Dąbrowski. Reactive oxygen species in photodynamic therapy: Mechanisms of their generation and potentiation. Adv. Inorg. Chem. 2017; 70, 343-94.
SD Astuty, Suhariningsih, A Baktir and SD Astuti. The efficacy of photodynamic inactivation of the diode laser in inactivation of the Candida albicans biofilms with exogenous photosensitizer of papaya leaf chlorophyll. J. Lasers Med. Sci. 2019; 10, 215-24.
PL Lam, RSM Wong, KH Lam, LK Hung, MM Wong, LH Yung, YW Ho, WY Wong, DKP Hau, R Gambari and CH Chui. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem. Biol. Interact. 2020; 320, 109023.
A Xie, H Li, Y Hao and Y Zhang. Tuning the toxicity of reactive oxygen species into advanced tumor therapy. Nanoscale Res. Lett. 2021; 16, 142.
F Collin. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019; 20, 2407.
CA Juan, JMPDL Lastra, FJ Plou and E Pérez-Lebeña. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021; 22, 4642.
M Morales and S Munné-Bosch. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019; 180, 1246-50.
L Sheng, X Li and L Wang. Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends Food Sci. Tech. 2022; 124, 167-81.
M Gallardo-Villagrán, DY Leger, B Liagre and B Therrien. Photosensitizers used in the photodynamic therapy of rheumatoid arthritis. Int. J. Mol. Sci. 2019; 20, 3339.
Y Li, X Zhang and D Liu. Recent developments of perylene diimide (PDI) supramolecular photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2021; 48, 100436.
MCS Vallejo, NMM Moura, MAF Faustino, A Almeida, I Gonçalves, VV Serra and MGPMS Neves. An insight into the role of non-porphyrinoid photosensitizers for skin wound healing. Int. J. Mol. Sci. 2021; 22, 234.
J Shen, Q Liang, G Su, Y Zhang, Z Wang, C Baudouin and A Labbé. In vitro effect of toluidine blue antimicrobial photodynamic chemotherapy on Staphylococcus epidermidis and Staphylococcus aureus isolated from ocular surface infection. Translational Vis. Sci. Tech. 2019; 8, 45.
E Polat and K Kang. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021; 9, 584.
HA Choi, DE Cheong, HD Lim, WH Kim, MH Ham, MH Oh, Y Wu, HJ Shin and GJ Kim. Antimicrobial and anti-biofilm activities of the methanol extracts of medicinal plants against dental pathogens streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017; 27, 1242-8
SD Astuti, PS Puspita, AP Putra, AH Zaidan, MZ Fahmi, A Syahrom and Suhariningsih. The antifungal agent of silver nanoparticles activated by diode laser as light source to reduce C. albicans biofilms: An in vitro study. Lasers Med. Sci. 2019; 34, 929-37.
TO Peulen and KJ Wilkinson. Diffusion of nanoparticles in a biofilm. Environ. Sci. Tech. 2011; 45, 3367-73.
L Xu, YY Wang, J Huang, CY Chen, ZX Wang and H Xie. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020; 10, 8996-9031.
A Gibała, P Żeliszewska, T Gosiewski, A Krawczyk, D Duraczyńska, J Szaleniec, M Szaleniec and M Oćwieja. Antibacterial and antifungal properties of silver nanoparticles-effect of a surface-stabilizing agent. Biomolecules 2021; 11, 1481.
M Piksa, C Lian, IC Samuel, KJ Pawlik, IDW Samuel and K Matczyszyn. The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 2023; 52, 1697-722.
N Rasiukevičiūtė, A Brazaitytė, V Vaštakaitė-Kairienė, A Kupčinskienė, P Duchovskis, G Samuolienė and A Valiuškaitė. The effect of monochromatic LED light wavelengths and photoperiods on botrytis cinerea. J. Fungi 2021; 7, 970.
MM Kim and A Darafsheh. Light sources and dosimetry techniques for photodynamic therapy. Photochem. Photobiol. 2020; 96, 280-94.
A Mirfasihi, BM Afzali, HE Zadeh, K Sanjari and M Mir. Effect of a combination of photodynamic therapy and chitosan on Streptococcus mutans (an in vitro study). J. Lasers Med. Sci. 2020; 11, 405-10.
SD Astuti, IB Utomo, EM Setiawatie, M Khasanah, H Purnobasuki, D Arifianto and KA Alamsyah. Combination effect of laser diode for photodynamic therapy with doxycycline on a wistar rat model of periodontitis. BMC Oral Health 2021; 21, 80.
S Ramadhana, SD Astuty, B Bannu, E Enggrianti and RR Wahyudi. Physicochemical study of castor leaf (Jatropha curcas L.) and lapaya leaf (Carica papaya L.) extract and their application as photosensitizer agents in antimicrobial photodynamic therapy system to Staphylococcus epidermidis biofilm. AIP Conf. Proc. 2023; 2719, 020018.
M Zain, SD Astuty, S Dewang, B Armynah, RR Wahyudi and S Ramadhana. Analysis of the changes power output and energy dose to green laser against OD and MDA values after photoinactivation at Candida albicans and Staphyloccocus epidermidis associate biofilms. AIP Conf. Proc. 2023; 2719, 020006.
SD Astuty, S Dewang, FA Tasmara and RR Wahyudi. Activity detection of noni leaf (Morinda citrifolia L.) chlorophyll extract through the photoinactivation to reducing Streptococcus mutans cells using XTT assay method. AIP Conf. Proc. 2022; 2663, 6.
PS Yerragopu, S Hiregoudar, U Nidoni, KT Ramappa, AG Sreenivas and SR Doddagoudar. Chemical synthesis of silver nanoparticles using tri-sodium citrate, stability study and their characterization. Int. Res. J. Pure Appl. Chem. 2020; 21, 37-50.
EN Gecer, R Erenler, C Temiz, N Genc and I Yildiz. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Particulate Sci. Tech. 2022; 40, 50-7.
H Zhang, M Peng, T Cheng, P Zhao, L Qiu, J Zhou, G Lu and J Chen. Silver nanoparticles-doped collagen-alginate antimicrobial biocomposite as potential wound dressing. J. Mater. Sci. 2018; 53, 14944-52.
IS Mfouo-Tynga, LD Dias, NM Inada and C Kurachi. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodynamic Ther. 2021; 34, 102091.
EV Maytin, U Kaw, M Ilyas, JA Mack and B Hu. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: A bilaterally controlled comparison study. Photodiagnosis Photodynamic Ther. 2018; 22, 7-13.
Z Jamali, SM Hejazi, SM Ebrahimi, H Moradi-Sardareh and M Paknejad. Effects of LED-based photodynamic therapy using red and blue lights, with natural hydrophobic photosensitizers on human glioma cell line. Photodiagnosis Photodynamic Ther. 2018; 21, 50-4.
LDC Leonel, ML Carvalho, BMD Silva, S Zamuner, C Alberto-Silva and MS Costa. Photodynamic antimicrobial chemotherapy (PACT) using methylene blue inhibits the viability of the biofilm produced by Candida albicans. Photodiagnosis Photodynamic Ther. 2019; 26, 316-23.
I Ahamad, F Bano, R Anwer, P Srivastava, R Kumar and T Fatma. Antibiofilm activities of biogenic silver nanoparticles against Candida albicans. Front. Microbiol. 2022; 12, 741493.
M Jalal, MA Ansari, MA Alzohairy, SG Ali, HM Khan, A Almatroudi and MI Siddiqui. Anticandidal activity of biosynthesized silver nanoparticles: effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomedicine 2019; 14, 4667-79.
MMJ Arsène, PI Viktorovna, M Alla, M Mariya, SA Nikolaevitch, AKL Davares, ME Yurievna, M Rehailia, AA Gabin, KA Alekseevna, YN Vyacheslavovna, ZA Vladimirovna, O Svetlana and D Milana. Antifungal activity of silver nanoparticles prepared using Aloe vera extract against Candida albicans. Vet. World 2023; 16, 18-26.
T Bruna, F Maldonado-Bravo, P Jara and N Caro. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021; 22, 7202.
PR More, S Pandit, AD Filippis, G Franci, I Mijakovic and M Galdiero. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023; 11, 369.
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.






