Anti-Diabetic Effect of Okara Noodles on Streptozotocin-Nicotinamide Induced Diabetic Rats
DOI:
https://doi.org/10.48048/tis.2024.7428Keywords:
Okara noodle, Anti-diabetic effect, STZ-NA, Diabetes mellitus, RatsAbstract
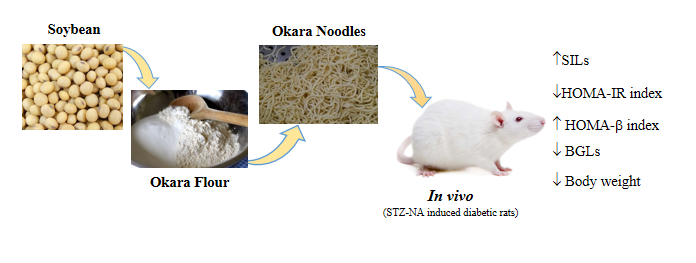
Okara is a by-product of soybean curd residue resulting from the processing of soy milk and tofu. Okara noodles were made from wheat flour (WF) and okara flour (OF) with a few modifications that consist of high dietary fiber. Therefore, okara can be considered as an effective functional component with health-promoting benefits, especially for diabetes mellitus. Albeit, the anti-diabetic effect has not been conscientiously investigated. The goal of the current research was to evaluate the anti-diabetic effect of okara noodles on Streptozotocin-Nicotinamide induced diabetes mellitus rats. In the experiment, the different ratios of OF was used to prepared 3 formulated noodles (F0, F1 and F2) where F0 (0 % OF; 100 % WF), F1 (25 % OF; 75 % WF) and F2 (45 % OF; 55 % WF). Twenty-four male wistar rats were randomly divided into 4 groups (6 rats/groups), normal group of rats fed with standard feed and 3 diabetics groups, respectively fed with standard feed and formulated noodles variety (F0, F1 and F2). In in vivo analysis, it indicated, diabetes mellitus rats fed okara noodles had lower blood glucose levels and HOMA-IR and higher serum insulin levels and HOMA-b index than those fed control noodles (F0) as well as body weight managing activity. These outcomes demonstrated okara noodles possess significant anti-diabetic activity, prompting the applicability of okara noodles as potential food for the diabetes mellitus food products.
HIGHLIGHTS
- Diabetes mellitus (DM) rats fed okara noodles had lower BGLs and HOMA-IR
- DM rats fed okara noodles had higher serum insulin levels and HOMA-b index than those fed control noodles as well as body weight managing activity
- Okara can be considered as an effective functional component for the anti-diabetic effect on STZ-NA induced DM rats
GRAPHICAL ABSTRACT

Downloads
References
A Al-Awar, K Kupai, M Veszelka, G Szucs, Z Attieh, Z Murlasits, S Török, A Pósa and C Varga. Experimental diabetes mellitus in different animal models. J. Diabetes Res. 2016; 2016, 9051426.
NPDC Subamia, KA Nocianitri and IDGM Permana. Utilization of tofu dregs flour in making snack bars for people with diabetes mellitus. Media Ilmiah Teknologi Pangan 2020; 7, 27-38.
KN Lesa, N Ahmad, Y Mayangsari, MU Khandaker, FMR Iqbal, DLN Fibri and MM Hassan. Health benefits of okara for the management of diabetes mellitus. J. Food Qual. 2023; 2023, 5540118.
R Goyal, I Jialal and M Castano. Type 2 diabetes (nursing). StatPearls, Florida, 2022.
SH Ahn, SC Oh, IG Choi, GS Han, HS Jeong, KW Kim, YH Yoon and I Yang. Environmentally friendly wood preservatives formulated with enzymatic-hydrolyzed okara, copper and/or boron salts. J. Hazard. Mater. 2010; 178, 604-11.
G Quintana, E Gerbino and A Gómez-Zavaglia. Okara: A nutritionally valuable by-product able to stabilize lactobacillus plantarum during freeze-drying, spray-drying, and storage. Front. Microbiol. 2017; 8, 641.
NG Forouhi and NJ Wareham. Epidemiology of diabetes. Medicine 2019; 47, 22-7.
AE Abdel-Mobdy, MS Khattab, EA Mahmoud, ER Mohamed and EA Abdel-Rahim. Semi-modified okara whey diet increased insulin secretion in diabetic rats fed a basal or high fat diet. Food Sci. Biotechnol. 2021; 30, 107-16.
B Giri, S Dey, T Das, M Sarkar, J Banerjee and SK Dash. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018; 107, 306-28.
MA Seid, Y Akalu, YY Gela, Y Belsti, M Diress, SA Fekadu, B Dagnew and M Getnet. Microvascular complications and its predictors among type 2 diabetes mellitus patients at Dessie town hospitals, Ethiopia. Diabetology Metab. Syndrome 2021; 13, 86.
R Kateel, AJ Augustine, S Prabhu, S Ullal, M Pai and P Adhikari. Clinical and microbiological profile of diabetic foot ulcer patients in a tertiary care hospital. Diabetology Metab. Syndrome 2018; 12, 27-30.
A Muhlishoh, B Wasita and AMP Nuhriawangsa. Antidiabetic effect of Centella asiatica extract (whole plant) in streptozotocin nicotinamide-induced diabetic rats. Jurnal Gizi Dan Dietetik Indonesia 2019; 6, 14-22.
SIR Okoduwa, IA Umar, DB James and HM Inuwa. Appropriate insulin level in selecting fortified diet-fed, streptozotocin-treated rat model of type 2 diabetes for anti-diabetic studies. PLos One 2017; 12, e0170971.
SK Reshmi, ML Sudha and MN Shashirekha. Noodles fortified with Citrus maxima (pomelo) fruit segments suiting the diabetic population. Bioactive Carbohydrates Diet. Fiber 2020; 22, 100213.
L Wang, L Wang, N Zhang, M Li and Z Li. Glucose metabolic effects of oat noodles with different processing in type 2 diabetic mice. J. Cereal Sci. 2019; 88, 125-31.
WC Vong and SQ Liu. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Tech. 2016; 52, 139-47.
DK O’Toole. Characteristics and use of okara, the soybean residue from soy milk production - a review. J. Agr. Food Chem. 1999; 47, 363-71.
WK Mok, YX Tan, J Lee, J Kim and WN Chen. A metabolomic approach to understand the solid-state fermentation of okara using Bacillus subtilis WX-17 for enhanced nutritional profile. AMB Express 2019; 9, 60.
S Huq, PC Das, MA Islam, MF Jubayer, TV Ranganathan and MAR Mazumder. Nutritional, textural, and sensory quality of oil fried donut enriched with extracted dietary fiber and okara flour. J. Food Process. Preservation 2021; 45, e15310.
MM Rahman, K Mat, G Ishigaki and R Akashi. A review of okara (soybean curd residue) utilization as animal feed: Nutritive value and animal performance aspects. Anim. Sci. J. 2021; 92, e13594.
N Ichikawa, LS Ng, S Makino, LL Goh, YJ Lim, Ferdinandus, H Sasaki, S Shibata and CLK Lee. Solid-state fermented okara with Aspergillus spp. improves lipid metabolism and high-fat diet induced obesity. Metabolites 2022; 12, 198.
B Li, M Qiao and F Lu. Composition, nutrition, and utilization of okara (soybean residue). Food Rev. Int. 2012; 28, 231-52.
MM Muliterno, D Rodrigues, FSD Lima, EI Ida and LE Kurozawa. Conversion/degradation of isoflavones and color alterations during the drying of okara. LWT Food Sci. Tech. 2017; 75, 512-9.
WC Pan, YM Liu and SY Shiau. Effect of okara and vital gluten on physico-chemical properties of noodle. Czech J. Food Sci. 2018; 36, 301-6.
ME Campderrós. Effect of okara flour addition on the physical and sensory quality of wheat bread. MOJ Food Process. Tech. 2017; 4, 184-90.
RK Grizotto, CRG Rufi, EA Yamada and E Vicente. Evaluation of the quality of a molded sweet biscuit enriched with okara flour. Food Sci. Tech. 2010; 30, 270-5.
X Hu, J Gao, Q Zhang, Y Fu, K Li, S Zhu and D Li. Soy fiber improves weight loss and lipid profile in overweight and obese adults: A randomized controlled trial. Mol. Nutr. Food Res. 2013; 57, 2147-54.
I Mateos-Aparicio, A Redondo-Cuenca, MJ Villanueva-Suárez, MA Zapata-Revilla and MD Tenorio-Sanz. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT Food Sci. Tech. 2010; 43, 1467-70.
JW Anderson, P Baird, RH Davis, S Ferreri, M Knudtson, A Koraym, V Waters and CL Williams. Health benefits of dietary fiber. Nutr. Rev. 2009; 67, 188-205.
L Jankowiak, O Trifunovic, RM Boom and AJVD Goot. The potential of crude okara for isoflavone production. J. Food Eng. 2014; 124, 166-72.
Y Cai, L Huang, X Tao, J Su, B Chen, M Zhao, Q Zhao and PVD Meeren. Carboxymethyl cellulose/okara protein influencing microstructure, rheological properties and stability of O/W emulsions. J. Sci. Food Agr. 2021; 101, 3685-92.
GL Wickramarathna and PC Arampath. Utilization of okara in bread making. J. Sci. 2003; 31, 29-33.
SA Zinia, A Rahim, MAL Jony, AA Begum and MAR Mazumder. The roles of okara powder on the processing and nutrient content of roti and paratha. Int. J. Agr. Environ. Sci. 2019; 6, 18-23.
DM Lebesi and C Tzia. Effect of the addition of different dietary fiber and edible cereal bran sources on the baking and sensory characteristics of cupcakes. Food Bioproc. Tech. 2011; 4, 710-22.
A Colletti, A Attrovio, L Boffa, S Mantegna and G Cravotto. Valorisation of by-products from soybean (Glycine max (L.) Merr.) processing. Molecules 2020; 25, 2129.
C Farrand, K Charlton, M Crino, J Santos, R Rodriguez-Fernandez, CN Mhurchu and J Webster. Know your noodles! assessing variations in sodium content of instant noodles across countries. Nutrients 2017; 9, 612.
F Eddyono and B Subroto. Purchase behavior of noodles: A case study of effort primary food diversification in Indonesia. Int. J. Sci. Tech. 2014; 3, 655-62.
WI Wan Rosli, AR Nurhanan and MS Aishah. Effect of partial replacement of wheat flour with oyster mushroom (pleurotus sajor-caju) powder on nutritional composition and sensory properties of butter biscuit. Sains Malaysiana 2012; 41, 1565-70.
Z Kohajdová, J Karovičová, M Magala and V Kuchtová. Effect of apple pomace powder addition on farinographic properties of wheat dough and biscuits quality. Chem. Paper. Chem. Zvesti 2014; 68, 1059-65.
PG Reeves, FH Nielsen and GC Fahey. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993; 123, 1939-51.
R Gupta and SB Sharma. Effect of germinated Glycine max seeds on glycemic control in STZ + NAD induced type 2 diabetic models: A preliminary study. J. Exp. Integr. Med. 2012; 2, 155.
SB Wahjuningsih, Haslina, S Untari and A Wijanarka. Hypoglycemic effect of analog rice made from modified cassava flour (Mocaf), arrowroot flour and kidney bean flour on STZ-NA induced diabetic rats. Asian J. Clin. Nutr. 2018; 10, 8-15.
D Barham and P Trinder. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972; 97, 142-5.
DR Nesti and A Baidlowi. Profil glukosa darah, lipid dan visualisasi pulau langerhans sebagai imunoreaktor insulin dan glukagon pada pankreas tikus (Rattus norvegicus) obesitas menggunakan teknik imunohistokimia. Jurnal Nasional Teknologi Terapan 2017; 1, 24-32.
IR Adriawan, M Andrie, R Susilowati, S Pramono and AE Nugroho. HOMA-IR index evaluation on antidiabetes mellitus effect of Andrographis paniculata (burm. f.) nees purified extract and andrographolide. Tradit. Med. J. 2014; 19, 19-23.
A Sutjahjo. High molecular weight adiponectin and vasculer thickness in diabetes type 2 related to fixed dose combination of glimepiride and metformin. Indonesian J. Clin. Pathol. Med. Lab. 2018; 21, 120-4.
E Nurus Sakinah, J Kalimantan No and K Tegalboto. The role of cholecalciferol in the improvement of insulin resistance in diabetic mice model. J. Agromedicine Med. Sci. 2017; 3, 24-9.
A Sutjahjo. Adiponektin high molecular weight dan kekakuan vaskular di penyakit diabetes melitus tipe 2 terkait gabungan glimepiride metformin dosis tetap. Indonesian J. Clin. Pathol. Med. Lab. 2015; 21, 120-4.
R Pramitasari, I Ivana and Yanti. Development of snack bar from black soybean and black rice for breastfeeding mothers. Int. J. Eng. Tech. 2018; 7, 288-91.
A Mohammed, NA Koorbanally and MS Islam. Ethyl acetate fraction of Aframomum melegueta fruit ameliorates pancreatic β-cell dysfunction and major diabetes-related parameters in a type 2 diabetes model of rats. J. Ethnopharmacol. 2015; 175, 518-27.
L Luo and M Liu. Adipose tissue in control of metabolism. J. Endocrinol. 2016; 231, R77-R99.
MS Burhans, DK Hagman, JN Kuzma, KA Schmidt and M Kratz. Contribution of adipose tissue inflammation to the development oftype 2 diabetes mellitus. Compr. Physiol. 2018; 9, 1-58.
C Chourpiliadis and SS Mohiuddin. Biochemistry, gluconeogenesis. StatPearls, Florida, 2022.
KM Sadek, MA Lebda, SM Nasr and M Shoukry. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017; 92, 1085-94.
E Farida, L Nuraida, PE Giriwono and BSL Jenie. Lactobacillus rhamnosus reduces blood glucose level through downregulation of gluconeogenesis gene expression in streptozotocin-induced diabetic rats. Int. J. Food Sci. 2020; 2020, 6108575.
T Szkudelski. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012; 237, 481-90.
A Aboonabi, A Rahmat and F Othman. Effect of pomegranate on histopathology of liver and kidney on generated oxidative stress diabetic induced rats. J. Cytol. Histol. 2014; 6, 294.
K Rehman, MSH Akash and Z Alina. Leptin: A new therapeutic target for treatment of diabetes mellitus. J. Cell. Biochem. 2018; 119, 5016-27.
Y Liu, S Yi, T Ye, Y Leng, MA Hossen, DE Sameen, J Dai, S Li and W Qin. Effects of ultrasonic treatment and homogenization on physicochemical properties of okara dietary fibers for 3D printing cookies. Ultrason. Sonochem. 2021; 77, 105693.
DB Kamble and S Rani. Bioactive components, in vitro digestibility, microstructure and application of soybean residue (okara): A review. Legume Sci. 2020; 2, e32.
HS Kim, OK Yu, MS Byun and YS Cha. Okara, a soybean by-product, prevents high fat diet-induced obesity and improves serum lipid profiles in C57BL/6J mice. Food Sci. Biotechnol. 2016; 25, 607-13.
F Lu, Y Liu and B Li. Okara dietary fiber and hypoglycemic effect of okara foods. Bioactive Carbohydrates Diet. Fiber 2013; 2, 126-32.
DJA Jenkins, A Marchie, LSA Augustin, E Ros and CWC Kendall. Viscous dietary fibre and metabolic effects. Clin. Nutr. Suppl. 2004; 1, 39-49.
RL Shen, FL Cai, JL Dong and XZ Hu. Hypoglycemic effects and biochemical mechanisms of oat products on streptozotocin-induced diabetic mice. J. Agr. Food Chem. 2011; 59, 8895-900.
V Gowd, L Xie, X Zheng and W Chen. Dietary fibers as emerging nutritional factors against diabetes: Focus on the involvement of gut microbiota. Crit. Rev. Biotechnol. 2019; 39, 524-40.
E Capuano. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017; 57, 3543-64.
H Zhang, S Sun and L Ai. Physical barrier effects of dietary fibers on lowering starch digestibility. Curr. Opin. Food Sci. 2022; 48, 100940.
D Mudgil and S Barak. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013; 61, 1-6.
YM Cassidy, EM McSorley and PJ Allsopp. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018; 46, 423-39.
D Feder and FLA Fonseca. Chapter 2 - The mechanism of fiber effects on insulin resistance. In: RA Samaan (Ed.). Dietary Fiber for the Prevention of Cardiovascular Disease. Academic Press, Massachusetts, 2017, p. 23-33.
MH Baky, M Salah, N Ezzelarab, P Shao, MS Elshahed and MA Farag. Insoluble dietary fibers: Structure, metabolism, interactions with human microbiome, and role in gut homeostasis. Crit. Rev. Food Sci. Nutr. 2022, https://doi.org/10.1080/10408398.2022.2119931.
B Svendsen and JJ Holst. Regulation of gut hormone secretion. Studies using isolated perfused intestines. Peptides 2016; 77, 47-53.
E Bahne, M Hansen, A Brønden, DP Sonne, T Vilsbøll and FK Knop. Involvement of glucagon-like peptide-1 in the glucose-lowering effect of metformin. Diabetes Obes. Metabol. 2016; 18, 955-61.
GM Ibrahim, OM Ahmed, NH Abbas and MME Fateh. Evaluation of the anti-diabetic effects of epicatechin and/or gallic acid in STZ/NA-induced diabetic Wister rats. Res. J. Appl. Biotechnol. 2018; 4, 87-104.
JM Siahaan, S Illyas, D Lindarto and M Nainggolan. The effect of ethanol and ethyl acetate fraction of chayote fruit (Sechium edule Jacq. Swartz) on the oxidative stress and insulin resistance of male white rat model type 2 diabetes mellitus. Open Access Macedonian J. Med. Sci. 2020; 8, 962-9.
AK Rines, K Sharabi, CDJ Tavares and P Puigserver. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016; 15, 786-804.
PV Röder, B Wu, Y Liu and W Han. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016; 48, e219.
MM Qaid and MM Abdelrahman. Role of insulin and other related hormones in energy metabolism-A review. Cogent Food Agr. 2016; 2, 1267691.
F Álvarez-Nava, D Bastidas, M Racines-Orbe and J Guarderas. Insulin sensitivity and pancreatic β-cell function in ecuadorian women with turner syndrome. Front. Endocrinol. 2020; 11, 482.
S Wen, C Liu, Y Li, J Pan, T Nguyen and L Zhou. Psoriasis exacerbates the state of insulin resistance in patients with type 2 diabetes. Diabetes Metab. Syndrome Obes. Targets Ther. 2021; 14, 2389-97.
F Zaccardi, DR Webb, T Yates and MJ Davies. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. 2016; 92, 63-9.
K Okita, H Iwahashi, J Kozawa, Y Okauchi, T Funahashi, A Imagawa and I Shimomura. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr. J. 2013; 60, 283-90.
MJ Oza and YA Kulkarni. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018; 107, 1119-27.
J Boucher, A Kleinridders and CR Kahn. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harbor Perspect. Biol. 2014; 6, a009191.
TC Chen, DI Benjamin, T Kuo, RA Lee, ML Li, DJ Mar, DE Costello, DK Nomura and JC Wang. The glucocorticoid-Angptl4-ceramide axis induces insulin resistance through PP2A and PKCz. Sci. Signaling 2017; 10, eaai7905.
H Khodabandehloo, S Gorgani-Firuzjaee, G Panahi and R Meshkani. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Translational Res. 2016; 167, 228-56.
H Wang, Y Lu, Y Yan, S Tian, D Zheng, D Leng, C Wang, J Jiao, Z Wang and Y Bai. Promising treatment for type 2 diabetes: Fecal microbiota transplantation reverses insulin resistance and impaired islets. Front. Cell. Infec. Microbiol. 2020; 9, 455.
M Lytrivi, AL Castell, V Poitout and M Cnop. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 2020; 432, 1514-34.
C Chen, CM Cohrs, J Stertmann, R Bozsak and S Speier. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol. Metabol. 2017; 6, 943-57.
DL Eizirik, L Pasquali and M Cnop. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020; 16, 349-62.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.






