Synthesis and Characterization of TiO2-Chitosan Beads and Its Application as A Degradation Agent of Methylene Blue
DOI:
https://doi.org/10.48048/tis.2023.6670Keywords:
Photocatalyst, Microwave, TiO2, Chitosan, Beads, Methylene blueAbstract
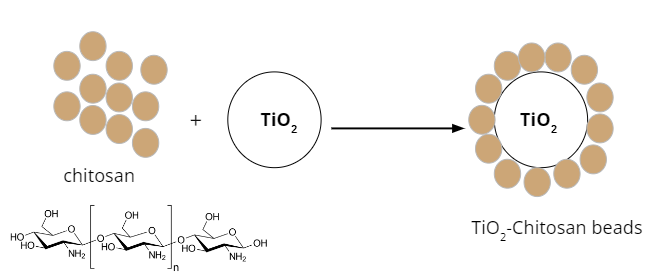
Dye waste is an environmental problem that has a negative impact on the ecosystem. One way to overcome this is by chemically degrading dyes using photocatalyst materials. TiO2 is one of the photocatalysts that can be synthesized by utilizing microwave radiation which is transferred directly to the material. This method takes a short amount of time so it can be completed in a very short time. TiO2 powder has the disadvantage that it is difficult to separate from the waste solution. TiO2 can be modified into beads by utilizing chitosan to produce TiO2-chitosan beads (TCB). In this research, the synthesis of TiO2 was carried out using microwave, followed by modification with chitosan to produce TCB and then its application in the degradation of methylene blue dye waste at various pH, concentration and contact time variations. Material characterization was carried out by determining the surface area of the material using Brunauer-Emmet-Teller (BET), band gap energy with UV-Vis Diffuse Reflectance Spectrocopy (UV-Vis DRS), crystal phase with X-Ray Diffraction (XRD), and surface morphology with Scanning Electron Microscope (SEM). The results showed that the synthesized TiO2 has anatase crystalline phase with an average crystal size of 18.85 nm and a bandgap energy of 3.16 eV. The TCB surface morphology indicated the successful impregnation of TiO2 on the chitosan. This study showed that the optimum performance of TCB was at pH 11 within 5 min and kinetic analysis results follows 2nd order.
HIGHLIGHTS
- Methylene blue dye waste pollutes the aquatic environment
- TiO2 as a photocatalyst can degrade methylene blue
- Chitosan is an abundant natural polymer which has high adsorption ability
- TiO2-chitosan beads material was synthesized to facilitate the separation of the material from the liquid after photodegradation process
GRAPHICAL ABSTRACT
Downloads
Metrics
References
SS Muthu. (2017). Introduction. In: SS Muthu (Ed.). Sustainability in the textile industry. Springer, Singapore, 2017.
P Sharma, N Capalash and V Gupta. Laccases and their role in bioremediation of industrial effluents. In: R Chandra (Ed.). Advances in biodegradation and bioremediation of industrial waste. CRC Press, Boca Raton, 2015.
D Pathania, S Sharma and P Singh. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arabian J. Chem. 2017; 10, S1445-S1451.
Y Pang, ZH Tong, L Tang, YN Liu and K Luo. Effect of humic acid on the degradation of methylene blue by peroxymonosulfate. Open Chem. 2018; 16, 401-6.
L Sun, D Hu, Z Zhang and X Deng. Oxidative degradation of methylene blue via PDS-based advanced oxidation process using natural pyrite. Int. J. Environ. Res. Publ. Health 2019; 16, 4773.
AT Farrel. Treatment of adults and children with acquired methemoglobinemia. Center for Drug Evaluation and Research Application, Maryland, 2015.
EA Abdelrahman, RM Hegazey and RE El-Azabawy. Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Tech. 2019; 8, 5301-13.
AH Jawad, AS Abdulhameed and MS Mastuli. Acid-factionalized biomass material for methylene blue dye removal: A comprehensive adsorption and mechanism study. J. Taibah Univ. Sci. 2020; 14, 305-13.
OE Ebi, AF Taiwo and AT Folorunsho. Kinetic modelling of the biosorption of methylene blue onto wild melon (Lagenariasphaerica). Am. J. Chem. Eng. 2018; 6, 126-34.
S Phanichphant, A Nakaruk, K Chansaenpak and D Chennai. Evaluating the photocatalytic efficiency of the BiVO4/rGO photocatalyst. Nat. Sci. Rep. 2019; 9, 16091.
G Sodeifian and R Behnood, R. Hydrothermal Synthesis of N-Doped GQD/CuO and N-Doped GQD/ZnO Nanophotocatalysts for MB dye removal under visible light irradiation: Evaluation of a new procedure to produce N-Doped GQD/ZnO. J. Inorg. Organomet. Polym. Mater. 2020; 30, 1266-80.
HNC Dharma, J Jaafar, N Widiastuti, H Matsuyama, S Rajabsadeh, MHD Othman, MA Rahmad, NNM Jafri, NS Suhaimin, AM Nasir and NH Alias. A review of titanium dioxide (TiO2-based photocatalyst for oilfield-produced water treatment. Membranes 2022; 12, 345.
CB Anucha, I Altin, E Bacaksiz and Stathopoulos. Titanium dioxide (TiO₂)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022; 10, 100262.
R Li, T Li and Q Zhou. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment - a review. Catalysts 2020; 10, 804.
G Li, L Lv, H Fan, J Ma, Y Li, Y Wan and XS Zhao. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010; 348, 342-7.
H Dong, G Zeng, L Tang, C Fan, C Zhang, X He and Y He. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015; 79, 128-46.
W Buraso, V Lachom, P Siriya, and P Laokul. Synthesis of TiO2 nanoparticles via a simple precipitation method and photocatalytic performance. Mater. Res. Exp. 2018; 5, 115003.
C Marien, C Marchal, A Koch, D Robert and P Drogui. Sol-gel synthesis of TiO2 nanoparticles: Effect of Pluronic P123 on particle’s morphology and photocatalytic degradation of paraquat. Environ. Sci. Pollut. Res. 2017; 24, 12582-8.
L Mardiana, AYP Wardoyo, Masruroh and HA Dharmawan. Synthesis TiO2 using sonochemical method and responses the CO2 gas of the nanoparticle TiO2 layers on the QCM sensor surfaces. J. Phys. Conf. Ser. 2022; 2165, 012014.
MP Izaak, YE Gunanto, H Sitompul and WA Adi. The synthesis of TiO2 nanoparticles by milling and coprecipitation mixed method. Jurnal Fisika Dan Aplikasinya 2020; 16, 67-70.
N Rab, FK Chong, HI Mohamed and WH Lim. Preparation of TiO2 nanoparticles by hydrolysis of TiCl4 using water and glycerol solvent system. J. Phys. Conf. Ser. 2018; 1123, 012065.
VM Ramakrishnan, M Natarajan, A Santhanam, V Asokan and D Velauthapillai. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018; 97, 351-60.
GS Falk, M Borlaf, MJ López-Muñoz, JC Fariñas, JBR Neto and R Moreno. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018; 20, 23.
EK Tetteh, S Rathilal, D Asante-Sackey and MN Chollom. Prospects of synthesized magnetic TiO2-based membranes for wastewater treatment: A review. Materials 2021; 14, 3524.
MC Kimling and RA Caruso. Sol-gel synthesis of hierarchically porous TiO2 beads using calcium alginate beads as sacrificial templates. J. Mater. Chem. 2012; 22, 4073-82.
G Calcagno, A Lotsari, A Dang, S Lindberg, AEC Palmqvist, A Matic and C Cavallo. Fast charging negative electrodes based on anatase titanium dioxide beads for highly stable Li-ion capacitors. Mater. Today Energ. 2020; 16, 100424.
G Tóth, F Bugyi, S Sugár, G Mitulović, K Vékey, L Turiák and L Drahoss. Selective TiO2 phosphopeptide enrichment of complex samples in the nanogram range. Separations 2020; 7, 74.
PS Bakshi, D Selvakumar, K Kadirvelu and NS Kumar. Chitosan as an environment friendly biomaterial - a review on recent modifications and applications. Int. J. Biol. Macromol. 2020; 150, 1072-83.
VP Santos, NSS Marques, PCSV Maia, MABD Lima, LO Franco and GMD Campos-Takaki. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020; 21, 4290.
A Wozniak and M Biernat. (2022), Methods for crosslinking and stabilization of chitosan structures for potential medical applications. J. Bioactive Compat. Polym. 2022; 37, 151-67.
C Tiloke, A Phulukdaree and AA Chuturgoon. The chemotherapeutic potential of gold nanoparticles against human carcinomas: A review. In: AM Holban and AM Grumezescu (Eds.). Nanoarchitectonics for smart delivery and drug targeting. William Andrew, New York, 2016, p. 783-811.
M Thommes, K Kaneko, A Neimark, JP Olivier, F Rodriguez-Reinoso, J Rouquerol and KSW Sing. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015; 87, 1051-69.
H Kaur, S Kumar, NK Verma and P Singh. Role of pH on the photocatalytic activity of TiO2 tailored by W/T mole ratio. J. Mater. Sci. Mater. Electron. 2018; 29, 16120-35.
P Atkins. Physical chemistry. Oxford University Press, London, 1999.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Walailak University

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.






